Spain to resume AstraZeneca vaccinations from Wednesday March 24th: health minister
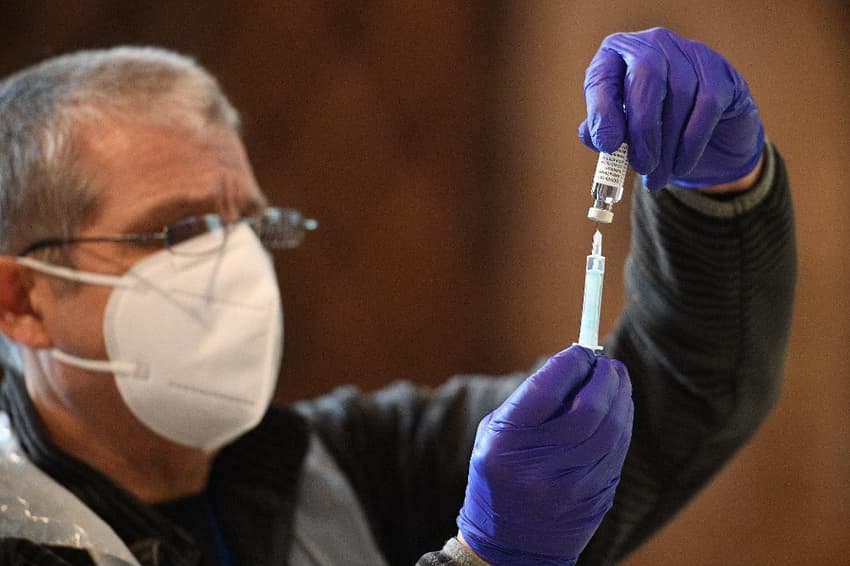
Spain said on Thursday it will resume its Covid-19 inoculation campaign with the AstraZeneca Covid-19 vaccine next week, after the EU's drug regulator deemed the jab "safe and effective".
Health Minister Carolina Darias told a news conference that Spain will start using the vaccine again "next week, namely Wednesday".
“The decision was taken unanimously as the benefits outweighed the risks, in line with the findings from the European Medicines Agency,” Darias told a news briefing after a meeting with Spain's regional health chiefs.
The unanimous decision was taken by Spain's Interterritorial Council of the National Health System (CISNS) after hearing the results from the investigation by the European Medicines Agency (EMA).
Ángela Domínguez, the coordinator of the vaccination program of the Spanish Epidemiology Society, emphasized that this process should give people confidence because it is a clear sign that "the pharmacovigilance system works".
The EMA’s Executive Director Emer Cooke said the agency’s expert committee came to “a clear and scientific conclusion”.
“This is a safe and effective vaccine whose benefits in protecting people from Covid-19 hugely outweigh the risks,” she said, but added that further studies would be conducted to rule out possible links between the injection and rare blood clotting cases.
Cooke said that the vaccine was not associated with an increased risk of thromboembolic events or blood clotting, but did add that based on the available evidence “we still cannot rule out definitely a link between these cases and the vaccine.”
In the report on its website the EMA said: “The vaccine may be associated with very rare cases of blood clots associated with thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it to clot) with or without bleeding, including rare cases of clots in the vessels draining blood from the brain (CVST)”.
Before vaccinations with AstraZeneca resume, Spain’s public health commission will meet again to discuss which groups the vaccine will be used on.
The country had been planning on extending the use of the AstraZeneca vaccine to the over 55s, due to more data being available on its effectiveness, however, they are now planning to review the EMA’s data to decide whether this plan will still go ahead. On Monday, March 22nd, the CISNS will make the final decision on this.
Earlier on Thursday, March 18th, a group of health experts at Oslo University Hospital concluded that the blood clots in three health workers who took the AstraZeneca vaccine were triggered by an immune system response.
Most of the blood clots that have been detected have been found in women around the age of 40.
The UK, which has administered more of the vaccine than other EU countries, has reported fewer such events than its neighbours, however.
While millions have been injected with the vaccine developed with Oxford University, small numbers of people have developed blood clots, which prompted Spain and other EU countries such as Germany, France, and Italy to suspend injections pending the EMA investigation.
READ MORE: Spain probes death of Marbella teacher after AstraZeneca jab
Comments
See Also
Health Minister Carolina Darias told a news conference that Spain will start using the vaccine again "next week, namely Wednesday".
“The decision was taken unanimously as the benefits outweighed the risks, in line with the findings from the European Medicines Agency,” Darias told a news briefing after a meeting with Spain's regional health chiefs.
The unanimous decision was taken by Spain's Interterritorial Council of the National Health System (CISNS) after hearing the results from the investigation by the European Medicines Agency (EMA).
Ángela Domínguez, the coordinator of the vaccination program of the Spanish Epidemiology Society, emphasized that this process should give people confidence because it is a clear sign that "the pharmacovigilance system works".
The EMA’s Executive Director Emer Cooke said the agency’s expert committee came to “a clear and scientific conclusion”.
“This is a safe and effective vaccine whose benefits in protecting people from Covid-19 hugely outweigh the risks,” she said, but added that further studies would be conducted to rule out possible links between the injection and rare blood clotting cases.
Cooke said that the vaccine was not associated with an increased risk of thromboembolic events or blood clotting, but did add that based on the available evidence “we still cannot rule out definitely a link between these cases and the vaccine.”
In the report on its website the EMA said: “The vaccine may be associated with very rare cases of blood clots associated with thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it to clot) with or without bleeding, including rare cases of clots in the vessels draining blood from the brain (CVST)”.
Before vaccinations with AstraZeneca resume, Spain’s public health commission will meet again to discuss which groups the vaccine will be used on.
The country had been planning on extending the use of the AstraZeneca vaccine to the over 55s, due to more data being available on its effectiveness, however, they are now planning to review the EMA’s data to decide whether this plan will still go ahead. On Monday, March 22nd, the CISNS will make the final decision on this.
Earlier on Thursday, March 18th, a group of health experts at Oslo University Hospital concluded that the blood clots in three health workers who took the AstraZeneca vaccine were triggered by an immune system response.
Most of the blood clots that have been detected have been found in women around the age of 40.
The UK, which has administered more of the vaccine than other EU countries, has reported fewer such events than its neighbours, however.
While millions have been injected with the vaccine developed with Oxford University, small numbers of people have developed blood clots, which prompted Spain and other EU countries such as Germany, France, and Italy to suspend injections pending the EMA investigation.
READ MORE: Spain probes death of Marbella teacher after AstraZeneca jab
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.